Namid Krüger’s HydRed field experiment of 2021: Studying hydraulic redistribution, coexistence of deep alfalfa and shallow rooted herbs and connection of arbuscular mycorrhizal networks

Namid Krüger studies environmental sciences at the TUBS. He is a hobby botanist, involved into the scientists for future campaign and highly motivated student. In this blog, he writes about his field experiments he conducted in 2021 on a potentially highly relevant topic for future agricultural management: water provision from deep soil layers to shallow soils via deep rooting plants and arbuscular mycorrhizal fungi. For more information on the experiments and interesting other ongoing projects, visit www.isodrones.com.
Since 2020, Isodrones and the Institute for Geoecology are conducting field experiments together with Dr. Falko Feldmann (JKI) and Jun.-Prof. Matthias Bücker (Institute of Geophysics and extraterrestrial Physics, TUBS) at the Julius Kühn-Institute (JKI) in Braunschweig on an effect called ‘hydraulic redistribution’ (HR): When surface soil gets dry, deep-rooted plants (such as Alfalfa, Medicago sativa) can reach water stored in deeper soil layers, where water is still available. A portion of water taken up at depth can potentially leach out of the shallow roots into the dry shallow soil layer over night because of water potential gradients (dry soil ‘pulls’ water out of the shallow roots). Coexisting shallow-rooted plants (that cannot reach the deep water with their own roots) can profit from the use of this water and can be potentially saved from negative drought effects. This process has been shown in many scientific studies (Burgess, 2011; Caldwell et al., 1998; Jackson et al., 2000; Prieto et al., 2012; Priyadarshini et al., 2016) and might have implications for future agricultural and forest management practices.
In the experiments for my bachelor thesis in 2020, I used the hydrogen isotope deuterium as a tracer in a greenhouse experiment, but this time we wanted to have ‘natural’ field conditions to show if hydraulic redistribution could be useful in agriculture. In the greenhouse many variables are not comparable to outside (e.g., temperature, humidity) which influences the microbial activity and water and nutrient transport. The greenhouse conditions can be regulated well and there are less problems/variables then in the field but the value of HR for crops could be only quantitatively but not quantitatively proven under these setup & conditions.
So, for the experiment in 2021, we planted different shallow-rooted plant species (e.g., Goldlack, Erysimum cheiri; Studentenblume, Tagetes; Majoran, Origanum majorana) surrounding a deep-rooted species (Alfalfa, Medicago sativa) in circles (April 2021) and injected a deuterium tracer through irrigation tubes in the deep soil layer in the middle of each plot at the end of the experiment (September 2021). In total, we had 23 plots with different combinations of deep/shallow-rooters and control plots (Fig. 1-5). Specifically, or objective to see if and which shallow-rooted species will get water from the deep-rooted to hypothesize which aspects promote the effect of HR and water transport in general.





In this context we also were interested in the arbuscular mycorrhiza (AM) symbiosis which is known to be important for water and nutrient supply and transport in the plant-soil interface and between connected plants (Poca et al., 2019). This is currently a ‘hot topic’ in the ecohydrological community because these fungi exist essentially in any environment of the world and have been shown to affect water and nutrient transport in many ways (Allen, 2007).
Results
Effect of Arbuscular mycorrhizal fungi
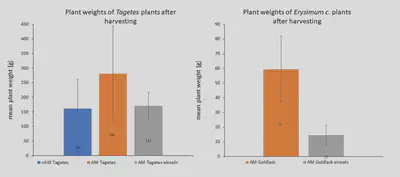
In the field experiment, we achieved a good colonization rate of the roots by putting AM inoculum in some plots (Fig8). In other words, the AMF connected very well with the plants. However, most of the plots without artificial AM addition were also full of naturally occuring AM, so the comparison between non-AM-plots and AM-plots was not conclusive. Despite that, it was particularly surprising how well the mycorrhization of Erysimum c. (Goldlack) worked. This species has previously been considered as not connecting with AMF. When looking at the weights of the harvested (shallow-rooting) plants after the experiment, there was a clear result: plants growing together with the deep rooting plant (Alfalfa) and a high degree of AMF population grew best (Fig9).
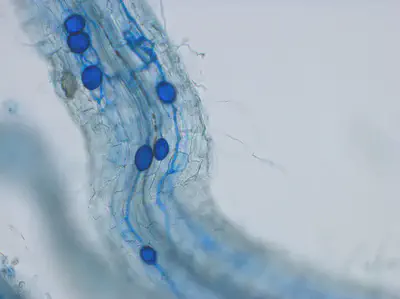
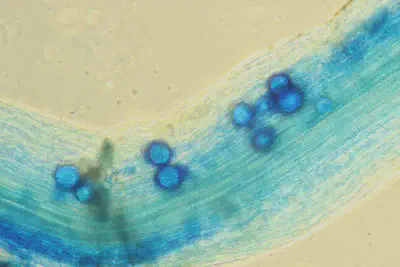
![Fig.8: Mycorrhization degree in [%]. For almost all treatments and plants, a good myccorhizatio nwas achieved.](/post/2022-02-04-hydred-field-experiment2021/Fig8_hu2b9cd2826e6883a12d8221793d10ca8c_65582_445adf9f01b3d22ea6da70525a02a2a1.webp)
Isotopic labeling and hydraulic redistribution
A big problem evolved at the end of the experiment. We decided to let the plants grow some weeks longer because the alfalfa plants weren’t so deep grown at all and we don’t want to insert the tracer less deep to don’t risk that the shallow-rooted plants can achieve it. We waited and waited for a good point in time with very dry conditions to start the tracer experiment and watch the HR happen. But the summer turned to a very rainy one and the autumn was coming soon….
As quick fix, we tried to achieve more dry conditions through the use of a foil but it didn’t work at all. Then, we decided to start our tracer experiment at a medium dry level because we thought conditions won’t get better. So, we started the tracer-injection under non-ideal conditions. We also conducted measured water isotopes in situ with a laser spectrometer in order to see if the highly enriched tracer is transpired by the target plants. In addition, some colleges conducted geophysical measurements to provide a picture of the specific electrical resistivity of the soil-root-system.

The next day when we took plant samples the second time to see if the tracer was transported through the root system into the shallow-rooted plants over night the conditions get wetter and wetter. Fortunately, we collected all samples before the heavy rain started.
The extraction of the plant samples we had taken from the xylem of the plants showed that there was no transport from the deep- to the shallow-rooted plants under these conditions over night. Some of the alfalfa plants took up some tracer water but the data showed a heterogenic pattern which is probably a result of differences in the rooting depth of individual alfalfa plants and heterogeneities of the soil. This experiment taught me how difficult it is to disentangle scientific processes directly in the field, in particular the highly sensible aspects we were looking at. Also, in this particular experiment, we were extremely unlucky in terms of the weather conditions. However, the experiment showed that the principle and methods we tested are suitable for such studies, and that motivates me for my upcoming Master Thesis experiments! Maybe a greenhouse experiment is easier to handle next time and also plants whith a shorter vegetation cycle are more practical so experiments can be done again when something happens unscheduled 😊
Isodrones thanks the Julius-Kühn Institute for providing the infrastructure and space for this experiment. We also thank Dr. Falko Feldmann for his valuable insights on mycorrhizal fungi and enthusiasm for city-greens. We further thank Dr. Matthias Bücker and his group at the IGEP for the collaboration, which will hopefully be only the beginning.
Further reading
Allen, M. F.: Mycorrhizal Fungi: Highways for Water and Nutrients in Arid Soils, Vadose Zo. J., 6(2), 291, doi:10.2136/vzj2006.0068, 2007.
Burgess, S. S. O.: Can hydraulic redistribution put bread on our table?, Plant Soil, 341(1–2), 25–29, doi:10.1007/s11104-010-0638-1, 2011.
Caldwell, M. M., Dawson, T. E. and Richards, J. H.: Hydraulic lift: consequences of water efflux from the roots of plants, Oecologia, 113(2), 151–161, doi:10.1007/s004420050363, 1998.
Jackson, R. B., Sperry, J. S. and Dawson, T. E.: Root water uptake and transport: using physiological processes in global predictions, Trends Plant Sci., 5(11), 482–488, doi:10.1016/S1360-1385(00)01766-0, 2000.
Poca, M., Coomans, O., Urcelay, C., Zeballos, S. R., Bodé, S. and Boeckx, P.: Isotope fractionation during root water uptake by Acacia caven is enhanced by arbuscular mycorrhizas, Plant Soil, 441(1–2), 485–497, doi:10.1007/s11104-019-04139-1, 2019.
Prieto, I., Armas, C. and Pugnaire, F. I.: Water release through plant roots: new insights into its consequences at the plant and ecosystem level., New Phytol., 193(4), 830–41, doi:10.1111/j.1469-8137.2011.04039.x, 2012.
Priyadarshini, K. V. R., Prins, H. H. T., de Bie, S., Heitkönig, I. M. A., Woodborne, S., Gort, G., Kirkman, K., Ludwig, F., Dawson, T. E. and de Kroon, H.: Seasonality of hydraulic redistribution by trees to grasses and changes in their water-source use that change tree-grass interactions, Ecohydrology, 9(2), 218–228, doi:10.1002/eco.1624, 2016.